|
|
|
|
|
|
|
|
|
Funding from following organizations are
gratefully acknowledged. |
|
|
|
|
   |
|

 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
On-Chip Development of Polymer Microfibers |
|
|
|
|
|
|
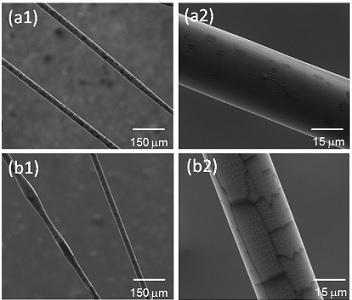
We use a microfluidic approach to fabricate biocompatible polymer fibers with
controlled sizes and cross sections.
Uniform gelatin microfibers with
various morphologies are fabricated by increasing the gelatin
concentration of core solution from 8% to 12%. Moreover, the increase
of gelatin concentration greatly improves the mechanical properties of
gelatin fibers; the Young’s modulus and tensile stress at break of
gelatin (12%) fiber are raised about 2.2 and 1.9 times as those of
gelatin (8%) fiber. The experiment results demonstrate that the
decrease of sheath-to-core flow rate ratio from 300:1 to 30:1 results in
the evolution of cross section from square to ribbon and the increase of
fiber dimension. The increased size and shape evolutions of cross
section from square to ribbon can not only strengthen the Young’s
modulus and tensile stress at break, and also significantly enhance
tensile strain at break.
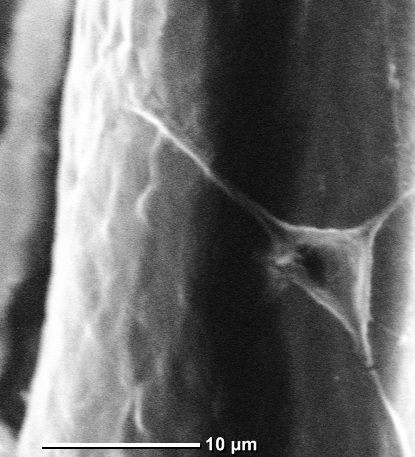
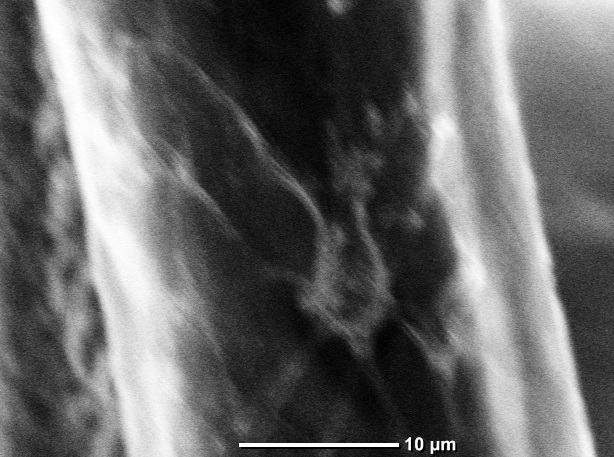
In addition, Adult Hippocampal Progenitor Cells (AHPCs) are shown to
successfully grow in vitro on PCL microfibers.
RSC Advances, 6, 55343-55353 (2016)
Journal of the Mechanical Behavior of Biomedical Materials, 61,
530-540 (2016)
RSC Advances, 5, 71203-71209 (2015)
Journal of Materials Chemistry A,
2, 4878-4884 (2014)
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biosensor for Optofluidic Characterization of Cells and
Particles |
|
|
|
|
|
|
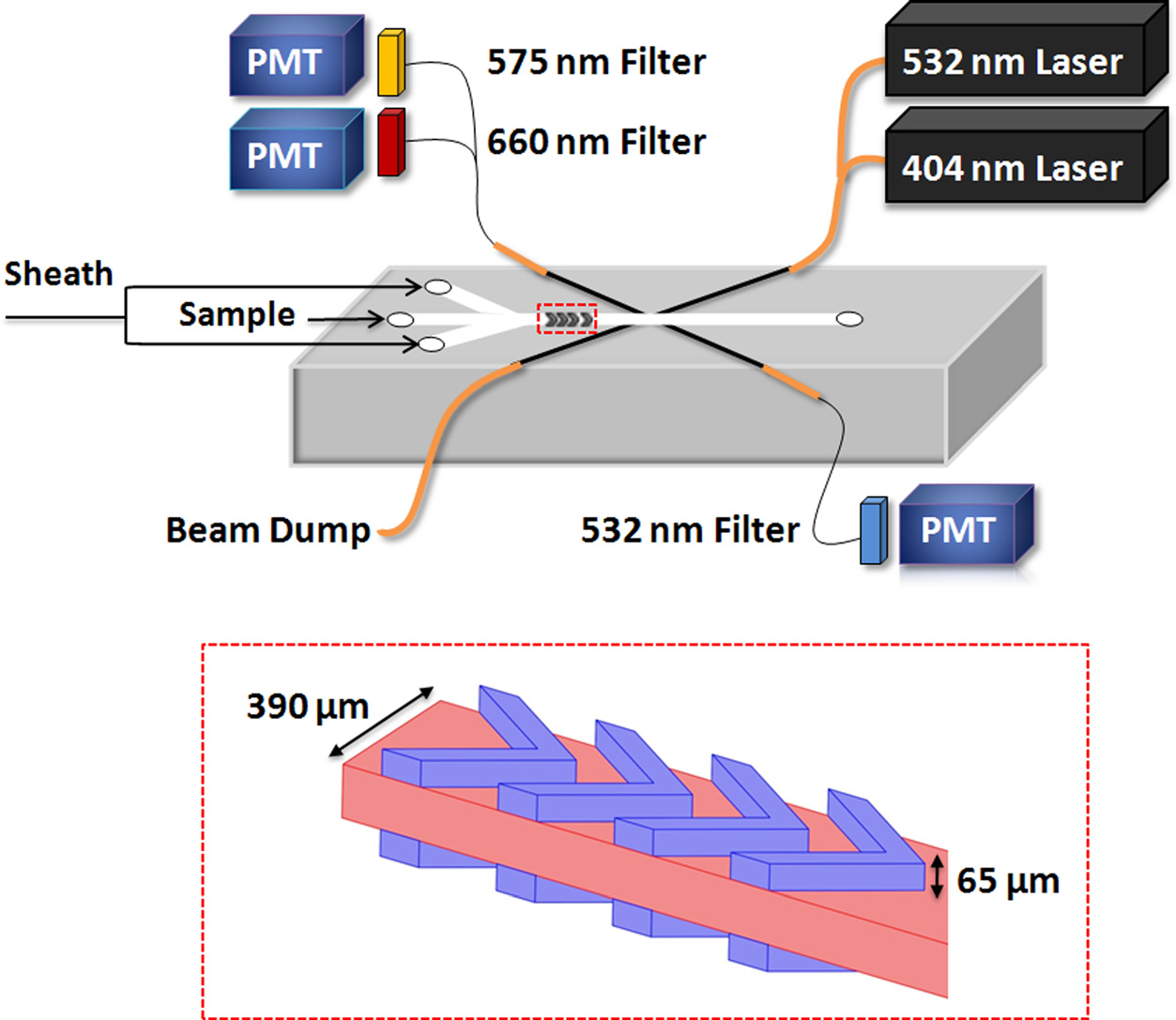
Analysis of the intrinsic fluorescence profiles of individual marine
algae can be used in general classification of organisms based on cell
size and fluorescence properties. We describe the design and fabrication
of a Microflow Cytometer on a chip for characterization of
phytoplankton. The Microflow Cytometer measures distinct side scatter
and fluorescence properties of Synechococcus sp., Nitzschia d., and
Thalassiosira p.; measurements are confirmed using the benchtop Accuri
C6 flow cytometer. The Microflow Cytometer is sensitive enough to
detect and characterize picoplankton with diameter approximately 1 μm
and larger phytoplankton of up to 80 μm in length. The wide range in
size discrimination coupled with detection of intrinsic fluorescent
pigments suggests that this Microflow Cytometer will be able to
distinguish different populations of phytoplankton on unmanned
underwater vehicles.
Biosensors, 5, 308-318 (2015)
Analytical Chemistry, 84, 839-850 (2012)
Biomicrofluidics, 5, 032009 (2011)
Biosensors and Bioelectronics, 26, 4263-4269 (2011)
Lab on a Chip,
10, 1952-1959 (2010)
Selected Press:
Most Read Articles in 2012 Published in Biomicrofluidics
NRC/ASEE Research Publication Award
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
Microfluidic Organ-on-a-Chip Technology for Advancement of Biological
Studies |
|
|
|
|
|
|
Drug testing targeted at the placenta has lacked reliable in vitro
testing designs to mimic in vivo situations. With the plethora
of different birth defects occurring around the world, attention needs
to be drawn to finding a potential alternative to testing live subjects.
Organ-on-a-chip technology has seen a vast increase in popularity, as
the understanding of utilizing the properties of microfluidics has
become more prevalent. Additionally, they are cost effective, use
minimal product to create, and dodge the ethical dilemma of using in
vivo animal models. Our goal is to create a microfluidic 3D cell culture
system representing a “placenta-on-a-chip” in order to mimic the
nutrient/waste transfer between maternal blood and fetal blood that
occurs in the cotyledon section of the placenta, and to test and observe
the effects of ethanol within the maternal bloodstream and compare it to
a similar in vivo situation.
Advanced Healthcare Materials, 4, 1426-1450 (2015)
Selected Press:
ISU biotech research attracts funding
Placenta-on-a-Chip: Universal Drug Testing Using Microfluidics
Mechanical engineering graduate student selected for international NSF
research program |
|
 |
|
|
|
|
|
|
|
|
|
|
|
Energy Microdevices |
|
|
|
|
|
|
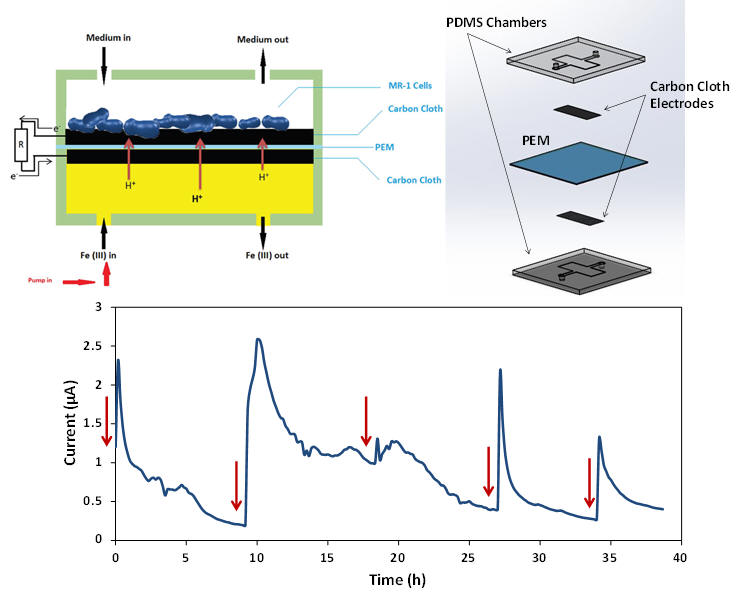
Miniature microbial fuel cells have recently drawn lots of attention as
portable power generation devices due to their short startup time and
environmentally-friendly process which could be used for powering small
integrated biosensors. We design and fabricate a microbial fuel cell
in a microfluidic platform. The device is made in polydimethylsiloxane
with a volume of 4 μL and consisted of two carbon cloth electrodes and
proton exchange membrane. Shewanella Oneidensis MR-1 is chosen to be
the electrogenic bacterial strain and inoculated into the anode chamber.
Ferricyanide is used as the catholyte and pumped into the cathode
chamber at a constant flow rate during the experiment. The miniature
microbial fuel cell generates a maximum current of 2.59 μA and has a
significantly short startup time.
Renewable & Sustainable Energy Review, 52, 1453-1472 (2015)
Physical Chemistry Chemical Physics, 15, 14147-14161 (2013)
Journal of
Applied Biosensor, 1, 21-25 (2012)
Selected Press:
Featured as Key Scientific Article on Renewable Energy Global
Innovations
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
3D Paper-Based Microfluidic Devices for Healthcare
Applications |
|
|
|
|
|
|
The first step in curing a disease is being
able to detect the disease effectively. The paper based microfluidic
devices are biodegradable and can make diagnosing diseases cost
effective and easy in almost all environments. We create a 3D paper
device using wax printing fabrication technique and basic principles of
origami. This design allows for a versatile fabrication technique over
previously reported patterning of SU-8 photoresist on chromatography
paper by employing readily available wax printer. The design also
utilizes multiple colorimetric assays which can accommodate one or more
analytes including urine, blood, and saliva. In this case to demonstrate
the functionality of the 3D paper based microfluidic system, a
urinalysis of protein and glucose assays is conducted. The amounts of
glucose and protein introduced to the device are found to be
proportional to the color change of each assay. This color change is
quantified using Adobe Photoshop. Urine samples from participants with
no pre-existing health conditions and one person with diabetes are
collected and compared against synthetic urine samples with
pre-determined glucose and protein levels. Utilizing this method, we
are able to confirm that both protein and glucose levels are in fact
within healthy ranges for healthy participants.
For participant with diabetes, the glucose is found to be above healthy
range while the protein level is in healthy range.
Renewable & Sustainable Energy Review, 52, 1453-1472 (2015)
Analytical Chemistry, 85, 10733–10737 (2013)
Selected Press:
Freshman finds success in SPEED
|
|
 |
|
|
 |
|
|
|
|
|
|
|
|
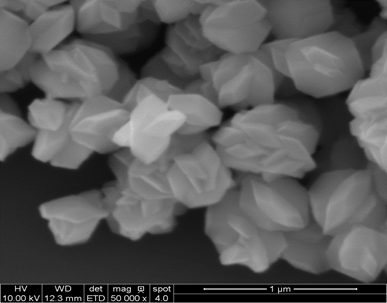
Development of MnF2
Nanocrystals and Graphene |
|
|
|
|
|
|
Upconverting Nanocrystals (UCNCs) are nanometer sized particles with the
ability to absorb low energy near infrared photons and emit higher
energy photons as near infrared and visible light. In cancer treatment,
UCNCs can dually enhance the contrast and selectivity of the imaging of
cancer cells in MRI as well as act as carriers for chemotherapy drugs
and photosensitizers for Photodynamic Therapy. MnF2 is
utilized for it’s quenching tendencies of green emissions and favor of
red emissions which is necessary for deep tissue penetration. Optical
abosorption in MnF2 is attributed to the d-d transisiton of
Mn2+ ions and fluoride contributes to low phonon energy which
increases UC efficiency and leads to powerful luminescence. In
comparison Yttrium Fluorides have shown to be some of the most efficient
host crystals for Upconversion luminescence and demonstrate a rather
simple synthesis on the nanometer scale.
Nanoscale, 7, 10101-10110 (2015)
RSC Advances, 4, 61891-61897 (2014)
Journal of Materials Chemistry C, 2, 1736-1741 (2014)
|
|
 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|













